Dalkon Shield
The article's lead section may need to be rewritten. (January 2022) |
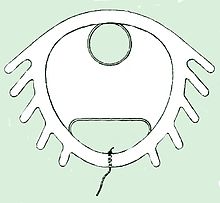
The Dalkon Shield was a contraceptive intrauterine device (IUD) developed by the Dalkon Corporation and marketed by the A.H. Robins Company. The Dalkon Shield was found to cause severe injury to a disproportionately large percentage of women, which eventually led to numerous lawsuits, in which juries awarded millions of dollars in compensatory and punitive damages.
Background
[edit]
The Dalkon shield was developed by Hugh J. Davis, a physician, and Irwin Lerner, an electrical engineer. Davis was a physician working as a gynecologist with an interest in limiting the effects of overpopulation in the world, as part of the Zero population growth theory popular in the 1960s.
He wrote: "While the upper socioeconomic tenth of the population can and does indulge in elaborate precoital rituals to control their fecundity the lowest socioeconomic tenth rejects such methods... The birth rate in the city of Baltimore in 1960 reflects this fact. There were 73 births per 1,000 white females in the highest economic class aged fifteen to forty-four, while in the lowest economic class there were 133 births. Thus, the segment of the population least able to discharge the responsibilities of parenthood was producing twice as many children per annum. The difference was nearly triple in the comparable non-white group."[1]
He setup a family planning clinic for the Johns Hopkins Hospital in 1964, with the help of the Ortho Pharmaceutical Company, with many of his public patients being poor and black Baltimoreans. By the late 1960s, they were inserting up to 70 IUDs a month, some experimental, created by Johns Hopkins and Ortho.[1]
In 1964, he began working with Irwin Lerner, an electrical engineer, on various medical devices, and over Christmas 1967, they decided to work on an IUD together. The design they came up with had a closed center (so as to not pose a risk of strangling the intestines if it migrated to the abdomen), and increase its surface area (and perhaps effectiveness) made of EVA (which had been approved by the FDA for use in food packaging), and fins were added to make it less likely to dislodge. This, in turn, made it more difficult to remove, and necessitated a stronger string, for which they used Supramid, a multifilament string encased in a nylon sheath.[1] Previous IUD designs had used a monofilament plastic string to reduce the chance of wicking bacteria from the non-sterile vaginal canal to the sterile uterus. To manage this risk, they tied a knot at either end of the string and ran experiments to see if inky water would wick past the knot and up the string. It did not, and no further wick testing was done by the company.[1]
Testing
[edit]Davis used the Johns Hopkins clinic to study the effects of his Dalkon shield on 640 women over a year-long period between 1968 and 1969. He reported a pregnancy rate of 1.1%, an expulsion rate of 2.3%, a retention rate of 94%, and reduced bleeding complications. However, this was later found to have numerous flaws; in the five months after the study was concluded, pregnancy rates were at 3-5%, it didn't have enough participants to give statistically significant results, and the average woman was enrolled for 5 months of the 12-month study; women who dropped out were not included in the results; some women were included after they had found success with the IUD, and Davis had recommended using spermicide during the most fertile days of patients cycles.[1]
Lerner applied for a patent alone in 1968, and Lerner, Davis, and their attorney Robert Cohen formed the Dalkon Corporation.[2][3][4]
After testing, the membrane of the shield was thinned and softened to help with extraction; copper sulfate was added to increase its radiopacity; metallic powdered copper was added to improve the plastic's strength; and they created a smaller version for women who had not given birth.[1]
The addition of copper sulfate caused a problem for the company, since at the time the FDA was obliged to study and approve medical drugs and not devices, and although the IUD as a whole was a medical device, the copper was ruled to be a drug. To evade oversight, Robins claimed the copper sulfate was only there to help with imaging.[1]
The effectiveness of these changes were not studied before the device went on the market.[1]
Marketing
[edit]In February 1970, Davis's study on his Dalkon Shield was published in the American Journal of Obstetrics and Gynecology. It did not mention his status as a co-inventor, or his financial interest in the device, nor was it peer-reviewed before publication. In the time between the study's submission to the journal and its publication, several women had become pregnant, making the pregnancy rate 3-5%, and taking it from better than other IUDs and oral contraceptives on the market to worst.[1]
The Dalkon Corporation in 1970 gained another investor, J. Earl, M.D., a medical practitioner in Defiance, Ohio.[5] Looking for a large retailer with marketing experience to sell their product, Earl met with a representative from A.H. Robins Company and sold them ownership rights and royalties. Robins was a pharmaceutical company but had no previous experience with birth control, nor had it made a medical device, an unregulated area at the time.[6]
The Dalkon Shield was promoted as a safer alternative compared to birth control pills, which at the time were the subject of many safety concerns.[5] Dr. Davis himself was a participant in the 1970 Nelson hearings, which were congressional hearings led by Senator Gaylord Nelson regarding the safety of oral contraceptives. He asserted that oral contraceptives with high doses of hormones were dangerous and that the efficacy of the pill was "greatly overrated".[7]
Release
[edit]In January 1971, Dalkon Shield went into the market, beginning in the United States and Puerto Rico, spearheaded by a large marketing campaign.[3][6] At its peak, about 2.8 million women used the Dalkon Shield in the U.S.
Design flaws
[edit]Bacterial wicking tail string
[edit]While looking for a material for the tail string, Davis and Learner discovered Supramid. Supramid was a cable-like suture material made of hundreds of fine inner nylon fibers encased by a smooth nylon outer sheath that was commonly used to repair tears in the tendons of horses. In 1971, a quality control supervisor found that the strings were able to wick water and suggested heat-sealing the ends of the string to form a barrier against wicking, but management rejected the idea as cost prohibitive. In addition to the string's ability to wick bacteria, the string also had a propensity to deteriorate inside the body, adding additional risks and giving bacteria another avenue to enter the string. These properties allowed bacteria to pass through the cervix into the uterus, bypassing the cervical mucus, which normally acts as a barrier against infection.[6][8][9][10]
Initial reports in the medical literature raised questions about whether its efficacy in preventing pregnancy and expulsion rate were as good as those claimed by the manufacturer, but failed to detect the tendency of the device to cause septic abortion and other severe infections.[11]
Painful and difficult insertions and removals
[edit]Physicians reported that the Dalkon Shield was more difficult to insert and painful than other types of IUDs, with a doctor writing to A.H. Robins in 1971 that "I have found the procedure to be the most traumatic manipulation ever perpetrated on womanhood, and I have inserted thousands of other varieties."[12]
The design of the insertion stick was also flawed, with the stick protruding past the end of the IUD. Physicians reported that it was easy to accidentally perforate the uterus with this tip.[1]
Infections
[edit]In June 1973, the Centers for Disease Control and Prevention (CDC) conducted a survey of 34,544 physicians with practices in gynecology or obstetrics regarding women who had been hospitalized or had died with complications related to the use of an IUD in the previous 6 months. A total of 16,994 physicians responded, yielding 3,502 unique case reports of women hospitalized in the first 6 months of 1973. Based on the survey response rate, the CDC estimated that a total of 7,900 IUD-related hospitalizations occurred during this 6-month period. Based on an estimate of 3.2 million IUD users, the CDC estimated an annual device-related hospitalization rate of 5 per 1000 IUD users. The survey also provided five reports of device-related fatalities, with four of these related to severe infections. One of the five was associated with the Dalkon Shield. Based on these data, the CDC estimated an IUD-related fatality rate of 3 per million users per year of use, which it compared favorably to the mortality risks associated with pregnancy and other forms of contraception. Importantly, the survey showed that the Dalkon Shield was associated with an increased rate of pregnancy-associated complications leading to hospitalization.[13]
By 1974, approximately 2.5 million women had received the Dalkon intrauterine device. In June of that year, the medical director of A.H. Robins published a letter to the editor of the British Medical Journal stating that the company was aware of an "apparent increase in the number of cases of septic abortions," including four fatalities, but stating that "there is no evidence of a direct cause-and-effect relationship between wearing of the Dalkon Shield and the occurrence of septicemia". The letter recommended precautions, including pregnancy tests for women who missed their period and the immediate removal of the device for women who were found to be pregnant.[14] That same month, A.H. Robins suspended sales of the device in the United States at the urging of the Food and Drug Administration, but continued to sell it overseas.[15] In October 1974, a series of four case reports of septic pregnancies were published in the journal Obstretics and Gynecology.[16] In 1975, the CDC published a study associating the Dalkon Shield with a higher risk of spontaneous abortion-related death compared to other IUDs.[17]
As many as 200,000 women made claims against the A.H. Robins company, mostly related to claims associated with pelvic inflammatory disease and loss of fertility. The company eventually filed for bankruptcy. The company's representatives argued that pelvic infections have a wide variety of causes, and that the Dalkon Shield was no more dangerous than other forms of birth control. Lawyers for the plaintiffs argued that the women they represented would be healthy and fertile today if not for the device. Scientists from the CDC stated that both arguments have merit.[18]
Company response
[edit]In 1971, 5 months after the IUD was released, the string was found to wick bacteria into the uterus by a quality control supervisor, and the company chose to change nothing.[6][19]
In June 1974, at the urging of the US FDA, Robins removed the IUD from the US market, but it continued to be marketed and sold internationally.[15][20]
In September 1980, Robins wrote a letter to physicians recommending they remove the Dalkon shield from women still wearing them.[19]
In 1984, 10 years after it was taken off the market in the US, the company put out newspaper, magazine, and television ads warning people of the risks and offering to pay for the shield's removal.[12]
Aftermath
[edit]Lawsuits and bankruptcy
[edit]More than 327,000 lawsuits and claims were filed against the A.H. Robins Company – the largest tort liability case since asbestos. The federal judge, Miles W. Lord, attracted public commentary for his judgments, holding the corporate heads personally accountable, saying "Your company in the face of overwhelming evidence denies its guilt and continues its monstrous mischief. You have taken the bottom line as your guiding beacon and the low road as your route. This is corporate irresponsibility at its meanest." [21][22][23]
The cost of litigation and settlements (estimated at billions of dollars) led the company to file for Chapter 11 bankruptcy protection in August 1985.[22]
The bankruptcy was legal but controversial at the time, as its assets were still sufficient to cover the current lawsuits and it was still making a profit, but by filing bankruptcy and creating a fixed-value trust fund for claimants, they were able to limit the damages paid.[24] As part of the bankruptcy, they set up a 2.5 billion-dollar trust fund for claimants. The share price went up following the bankruptcy, and the owners were later able to sell the A.H. Robins for a profit to American Home Products (now Wyeth) in 1989, with American Home Products receiving tax deductions up to about 1 billion dollars from the trust fund for victims and related to purchasing a company that has been in bankruptcy.[25]
More than half of the women in the class action suit were paid less than $1000, although some may have been paid out as much as $1 million in the cases of babies born with birth defects.[26]
Overseas Lawsuits
[edit]Overseas, the Dalkon shield continued to be marketed and sold for years after its withdrawal from the American market in 1974. In Australia, company memos informed their sales representatives of problems reported in the US, but this information was 'not to be used in doctor discussions'. It continued to be inserted as late as 1980.[24]
Australia
[edit]By 1989, over 7,000 Australians were suing the company in relation to pregnancies, pelvic inflammatory disease, ectopic pregnancies, spontaneous septic abortions, and perforated uteri.[20]
Legislative changes to the FDA
[edit]In 1976, the Medical Device Amendments to the Food, Drug, and Cosmetic Act mandated the U.S. Food and Drug Administration, for the first time, to require testing and approval of not just medications, but also medical devices, including IUDs.[27]
Effect on IUD sales
[edit]Pharmaceutical companies were discouraged from investing in IUDs after the bankruptcy of Robins, deciding that they were less profitable and more controversial than oral contraceptives. There were no IUDs on the market in the USA between 1983 and 1988, and newer IUDs remained unpopular through the 1990s, likely due to memories of the Dalkon Shield amongst both physicians and the populace.[12]
Only 1% of American women (aged 15-44) were using IUDs in 1995, but that number grew to 2.4% by 2004, and by 2017 the number had climbed to 14.0%, as a younger generation of women began embracing the newer IUDs as a safe, long-acting form of birth control.[28]
References
[edit]- ^ a b c d e f g h i j Perry & Dawson 1985.
- ^ Krismann, Carol H. (17 December 2015). "Dalkon Shield". Encyclopedia Britannica. Retrieved 2 January 2023.
- ^ a b Kolb, Robert W. (2018). The SAGE encyclopedia of business ethics and society. pp. 813–814. doi:10.4135/9781483381503. ISBN 978-1-4833-8151-0. OCLC 1030656249.
- ^ US patent 3633574, Lerner, "Intrauterine contraceptive device", published 1972-01-11, issued 1972-01-11
- ^ a b Robert McG., Thomas Jr. (October 26, 1996). "Hugh J. Davis, 69, Gynecologist Who Invented Dalkon Shield". The New York Times. Retrieved September 11, 2016.
- ^ a b c d Mintz, Morton (7 April 1985). "Questions Arose Early On Contraceptive's Safety". The Washington Post. Retrieved 2 January 2023.
- ^ United States (1967). Competitive problems in the drug industry: hearings before Subcommittee on Monopoly of the Select Committee on Small Business, United States Senate, Ninetieth Congress, first session ... Washington: U.S. Govt. Print. Off.
- ^ Siegel, Barry (August 22, 1985). "The Right Question : One Man's Effort to Tell Dalkon Story". Los Angeles Times. Retrieved April 30, 2023.
- ^ Sobol, R.B. (1993). Bending the Law: The Story of the Dalkon Shield Bankruptcy. University of Chicago Press. p. 2. ISBN 978-0-226-76753-6. Retrieved 30 April 2023.
- ^ C., Anna (March 28, 2016). ""Instrument of Torture": The Dalkon Shield Disaster". Planned Parenthood Advocates of Arizona.
- ^ Jones, R.W.; Parker, A.; Elstein, Max (1973). "Clinical experience with the Dalkon Shield intrauterine device". Br Med J. 3 (5872): 143–5. doi:10.1136/bmj.3.5872.143. PMC 1586323. PMID 4720765.
- ^ a b c Bahr, Anna (29 August 2012). "As Memories of Dalkon Shield Fade, Women Embrace IUDs Again". Ms Magazine. Retrieved January 25, 2024.
- ^ CDC (October 17, 1997). "Current trends IUD safety: report of a nationwide physician survey". MMWR. 46 (41): 969–974.
- ^ Templeton, J.S. (1974). "Letter: septic abortion and the Dalkon Shield". Br Med J. 2 (5919): 612. doi:10.1136/bmj.2.5919.612. PMC 1610760. PMID 4833976.
- ^ a b "Dalkon Shield Sale Halted by the Company". The New York Times. 29 June 1974. Retrieved 2 January 2023.
- ^ Hurt, W.G. (1974). "Septic pregnancy associated with Dalkon Shield intrauterine device". Obstet Gynecol. 44 (4): 491–5. PMID 4607058.
- ^ Cates, W.; Ory, H.W.; Rochat, R.W.; Tyler, C.W. (1976). "The intrauterine device and deaths from spontaneous abortion". N. Engl. J. Med. 295 (21): 1155–9. doi:10.1056/NEJM197611182952102. PMID 980018.
- ^ Kolata, Gina (December 6, 1987). "The sad legacy of the Dalkon Shield". The New York Times.
- ^ a b Mastroianni L Jr.; Donaldson PJ; Kane TT, eds. (1990). "Products Liability and Contraceptive Development". Products Liability and Contraceptive Development.
- ^ a b Cashman 1989, p. 92.
- ^ Serrill, Michael S. (July 23, 1984). "A Panel Tries to Judge a Judge". Time. Archived from the original on October 29, 2010.
- ^ a b Mintz, Morton (April 28, 1987). "Plan of A. H. Robins Would Pay Shield Victims as Little as $100". Washington Post. Retrieved April 30, 2023.
- ^ "JUDGE LAMBASTES COMPANY IN SUIT ON INTRAUTERINE DEVICE". The New York Times. March 2, 1984. Retrieved January 25, 2024.
- ^ a b "Dalkon shield". Public Interest Advocacy Centre.
- ^ Gladwell, Malcolm (15 December 1989). "American Home's 'Steal' of a Deal for A.H. Robins". Washington Post. Retrieved 30 April 2023.
- ^ Hicks 1994.
- ^ Adler, Robert (1988). "The 1976 Medical Device Amendments: A Step in the Right Direction Needs Another Step in the Right Direction". Food, Drug, Cosmetic Law Journal. 43 (3): 511–532. ISSN 0015-6361. JSTOR 26658558.
- ^ "Intrauterine Devices (IUDs): Access for Women in the U.S." KFF. 9 September 2020. Retrieved 27 June 2024.
Bibliography
[edit]- Szaller, Jim (Winter 1999). "One Lawyer's 25 Year Journey: The Dalkon Shield Saga". Ohio Trial. 9 (4). Archived from the original (Reprint) on 2006-05-13. Retrieved 2006-08-17. – Chronicles legal team of Brown & Szaller's involvement in the Dalkon Shield Litigation.
- Engelmayer, Sheldon D. (1985-09-22). "Lord's Justice : One Judge's War Against the Infamous Dalkon Shield" (New York Times Review). The New York Times. New York. ISBN 978-0-385-23051-3. Retrieved 2010-04-30.
- Speroff, L.; Glass, R.H.; Kase, N.G. (1999). Clinical Gynecological Endocrinology and Infertility (6th ed.). Lippincott, Williams & Wilkins. p. 976. ISBN 978-0-683-30379-7.
- Gordon, Meryl (February 20, 1999). "A Cash Settlement, But No Apology". New York Times. Retrieved 2006-08-17.
- Sivin, I. (1993). "Another look at the Dalkon Shield: meta-analysis underscores its problems". Contraception. 48 (1): 1–12. doi:10.1016/0010-7824(93)90060-k. PMID 8403900.
- Anselmi, Katherine Kaby (1994). Women's response to reproductive trauma secondary to contraceptive iatrogenesis: A phenomenological approach to the Dalkon Shield case (Abstract) (Thesis). pp. 1–226.
- "Robins Plan Is Approved". New York Times. Associated Press. June 17, 1989.
- Shereff, Ruth (February 13, 1989). "How to Reward The Criminals". The Nation. 248 (6).
Books
[edit]- Bacigal, Ronald J. (1990). The Limits Of Litigation: The Dalkon Shield Controversy. Durham, N.C.: Carolina Academic Press. ISBN 0890893918.
- Cashman, Peter (1989). "The Dalkon Shield". In Grabosky, Peter; Sutton, Adam (eds.). Stains on a white collar: fourteen studies in corporate crime or corporate harm. Sydney, Australia: Federation Press. p. 92. ISBN 1 86287 009 8.
- Grant, Nicole J. (1992). The Selling of contraception : the Dalkon Shield case, sexuality, and women's autonomy. Columbus: Ohio State University Press. ISBN 978-0814205723.
- Hawkins, Mary E. (1997). Unshielded: The Human Cost Of The Dalkon Shield. Toronto: University of Toronto Press. ISBN 0802008763.
- Hicks, Karen M. (1994). Surviving The Dalkon Shield Iud : Women v. The Pharmaceutical Industry. New York: Teachers College Press. ISBN 0807762717.
- Mintz, Morton (1985). At Any Cost: Corporate Greed, Women, And The Dalkon Shield. New York: Pantheon. ISBN 0394548469.
- Abstract: Morton Mintz (January 15, 1986). "A Crime Against Women: A.H. Robins and the Dalkon Shield". Multimedia Monitor. 7 (1). – Includes full text of presiding judge Miles Lord's statement to Clairbone Robins, et al., at bottom.
- Reviewed and summarised by: Tamar Lewin (1986-01-12). "What Standards For Corporate Crime?". New York Times.
- Perry, Susan & Dawson, Jim (1985). Nightmare: Women And The Dalkon Shield. New York: Macmillan. ISBN 0025959301.
- Stern, Gerald M. (1976). The Buffalo Creek Disaster. New York: Random House. ISBN 0394403908.
